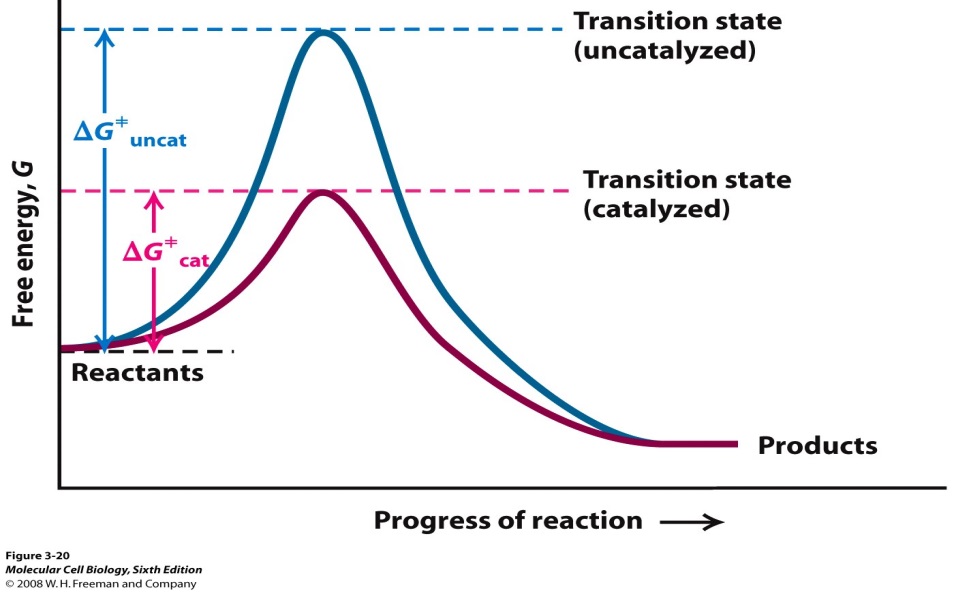
How Does Catalyst Affect The Rate Of Chemical Reaction . a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. So a low energy particle could, an instant later, have gained enough energy from a. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst does not actually become part of the. a catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. Adding a catalyst has this effect on activation energy. Catalysts play an important part in many chemical processes. Catalysts participate in a chemical reaction and increase its rate. some particles will gain energy in random collisions, and others will lose energy. in other words, to move the activation energy to the left on the graph: The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction.
from exohgcpop.blob.core.windows.net
The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. some particles will gain energy in random collisions, and others will lose energy. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst does not actually become part of the. a catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. Catalysts participate in a chemical reaction and increase its rate. Adding a catalyst has this effect on activation energy. So a low energy particle could, an instant later, have gained enough energy from a. Catalysts play an important part in many chemical processes. in other words, to move the activation energy to the left on the graph:
A Catalyst Increases The Rate Of Reaction As It at Gladys McCoy blog
How Does Catalyst Affect The Rate Of Chemical Reaction a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. a catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. They do not appear in the reaction’s net equation and are not consumed during the reaction. a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. in other words, to move the activation energy to the left on the graph: So a low energy particle could, an instant later, have gained enough energy from a. The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. some particles will gain energy in random collisions, and others will lose energy. A catalyst does not actually become part of the. Catalysts participate in a chemical reaction and increase its rate. Catalysts play an important part in many chemical processes. Adding a catalyst has this effect on activation energy.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction How Does Catalyst Affect The Rate Of Chemical Reaction Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. So a low energy particle could, an instant later, have gained enough energy from a. Adding a catalyst has this effect on activation energy. Catalysts play an important part in many chemical processes. . How Does Catalyst Affect The Rate Of Chemical Reaction.
From byjus.com
How does a catalyst increase the rate of a reaction? How Does Catalyst Affect The Rate Of Chemical Reaction They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. So a low energy particle could, an instant later, have gained enough energy from a. some particles will gain energy in random collisions, and others will lose energy. a catalyst is a. How Does Catalyst Affect The Rate Of Chemical Reaction.
From celimoko.blob.core.windows.net
Catalyst Reactions Examples at Jenny McNear blog How Does Catalyst Affect The Rate Of Chemical Reaction The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. some particles will gain energy in random collisions, and others will lose energy. in other words, to move the activation energy to the left on the graph: a catalyst is a substance which increases the rate of a chemical. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID How Does Catalyst Affect The Rate Of Chemical Reaction The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. So a low energy particle could, an instant later, have gained enough energy from a. a catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. some particles will gain. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.youtube.com
How does Catalyst affect the Rate of Chemical Reaction Lowering or How Does Catalyst Affect The Rate Of Chemical Reaction They do not appear in the reaction’s net equation and are not consumed during the reaction. in other words, to move the activation energy to the left on the graph: some particles will gain energy in random collisions, and others will lose energy. Catalysts participate in a chemical reaction and increase its rate. The addition of a catalyst. How Does Catalyst Affect The Rate Of Chemical Reaction.
From cemhahrt.blob.core.windows.net
How Does Catalysts Increase The Rate Of A Chemical Reaction at Gary How Does Catalyst Affect The Rate Of Chemical Reaction The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. So a low energy particle could, an instant later, have gained enough energy from a. in other words, to move the activation energy to the left on the graph: A catalyst does not actually become part of the. a catalyst. How Does Catalyst Affect The Rate Of Chemical Reaction.
From circuitdiagramlows.z22.web.core.windows.net
Catalyst Energy Diagram How Does Catalyst Affect The Rate Of Chemical Reaction The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. some particles will gain energy in random collisions, and others will lose energy. Adding a catalyst has this effect on activation. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation How Does Catalyst Affect The Rate Of Chemical Reaction The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. Adding a catalyst has this effect on activation energy. some particles will gain energy in random collisions, and others will lose energy. So a low energy particle could, an instant later, have gained enough energy from a. They do not appear. How Does Catalyst Affect The Rate Of Chemical Reaction.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions How Does Catalyst Affect The Rate Of Chemical Reaction in other words, to move the activation energy to the left on the graph: a catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. They do not appear in the reaction’s net equation and are not consumed during the reaction. some particles will gain energy in. How Does Catalyst Affect The Rate Of Chemical Reaction.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 How Does Catalyst Affect The Rate Of Chemical Reaction A catalyst does not actually become part of the. Adding a catalyst has this effect on activation energy. in other words, to move the activation energy to the left on the graph: The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. They do not appear in the reaction’s net equation. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.chemicals.co.uk
Chemistry GCSE Revision The Rate and Extent of Chemical Change How Does Catalyst Affect The Rate Of Chemical Reaction a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. A catalyst does not actually become part of the. in other words, to move the activation energy to the left on the graph: Catalysts participate in a chemical reaction and increase its rate. some particles will gain energy. How Does Catalyst Affect The Rate Of Chemical Reaction.
From ppt-online.org
Rates of reaction презентация онлайн How Does Catalyst Affect The Rate Of Chemical Reaction They do not appear in the reaction’s net equation and are not consumed during the reaction. The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. Adding a catalyst has this effect on activation energy. A catalyst does not actually become part of the. in other words, to move the activation. How Does Catalyst Affect The Rate Of Chemical Reaction.
From dxxaaiodeco.blob.core.windows.net
Catalyst Chemical Formula at Paul Lowther blog How Does Catalyst Affect The Rate Of Chemical Reaction So a low energy particle could, an instant later, have gained enough energy from a. a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts play an important part in many chemical processes.. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint How Does Catalyst Affect The Rate Of Chemical Reaction A catalyst does not actually become part of the. So a low energy particle could, an instant later, have gained enough energy from a. a catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. Catalysts participate in a chemical reaction and increase its rate. a catalyst is. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 How Does Catalyst Affect The Rate Of Chemical Reaction They do not appear in the reaction’s net equation and are not consumed during the reaction. some particles will gain energy in random collisions, and others will lose energy. in other words, to move the activation energy to the left on the graph: So a low energy particle could, an instant later, have gained enough energy from a.. How Does Catalyst Affect The Rate Of Chemical Reaction.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Does Catalyst Affect The Rate Of Chemical Reaction So a low energy particle could, an instant later, have gained enough energy from a. A catalyst does not actually become part of the. They do not appear in the reaction’s net equation and are not consumed during the reaction. a catalyst is a substance which increases the rate of a chemical reaction but it is not used up. How Does Catalyst Affect The Rate Of Chemical Reaction.
From philschatz.com
Catalysis · Chemistry How Does Catalyst Affect The Rate Of Chemical Reaction a catalyst is a substance that can help the reactants in a chemical reaction react with each other faster. So a low energy particle could, an instant later, have gained enough energy from a. The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. They do not appear in the reaction’s. How Does Catalyst Affect The Rate Of Chemical Reaction.
From cedydkgy.blob.core.windows.net
Effect Of Catalyst On Rate Of Reaction Example at Brittany Wagar blog How Does Catalyst Affect The Rate Of Chemical Reaction The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. Catalysts participate in a chemical reaction and increase its rate. A catalyst does not actually become part of the. some particles will gain energy in random collisions, and others will lose energy. in other words, to move the activation energy. How Does Catalyst Affect The Rate Of Chemical Reaction.